With XRF, most of the elements are only measured on the surface of the sample. This is why the condition of the sample surface with respect to smoothness and homogeneity (surface area and depth) is so essential. In practice, this more or less determines the total analytical error. Thus the sample preparation for XRF becomes the most important component of a test method.
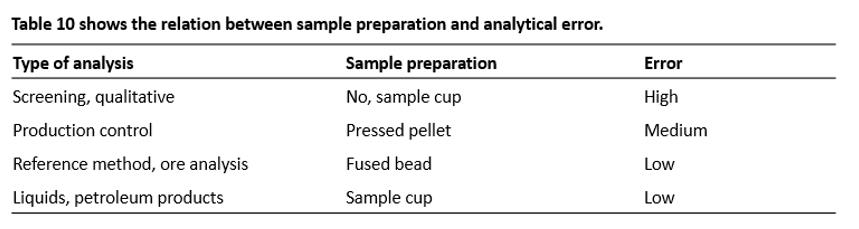
Figure 33 shows the five typical sample preparation forms for XRF:
· Solid sample prepared as a loose powder
· Solid sample prepared as pressed powder with or without binder
· Solid sample in its original form
· Liquid in a sample cup
· Solid sample prepared as a fused bead
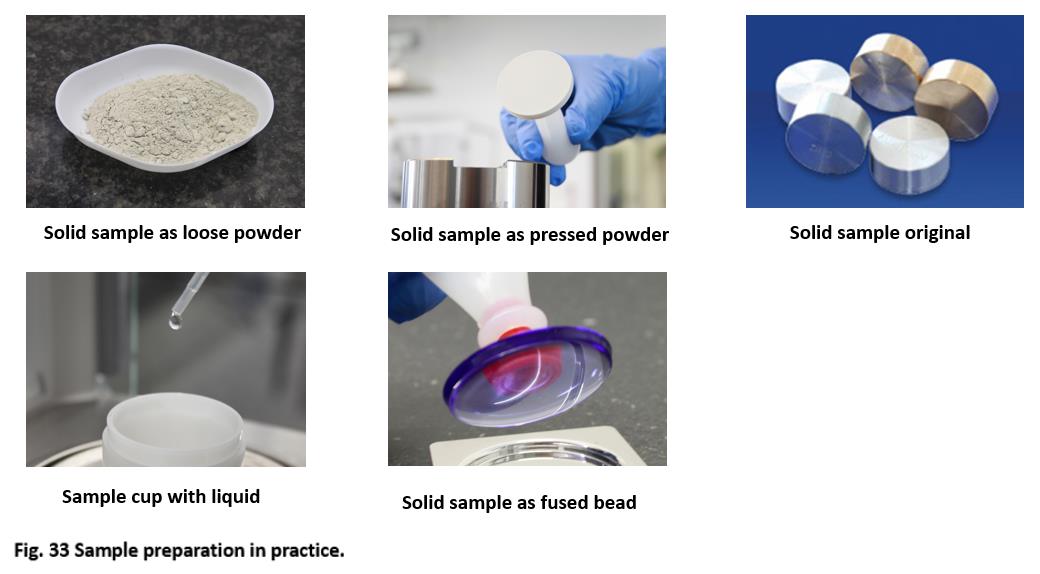
Table 10 shows that increasing sample preparation efforts always result in a lower analytical error. The analytical goal is the deciding factor for the choice of sample preparation. The main emphasis for rapid screening analyses, e.g., for pre-sorting of samples in the laboratory, is speed; not so much precision. In this case the samples are frequently measured in the original form as a piece or loose powder.
In contrast, a test method with high precision requires extensive sample preparation, such as, e.g., fusion for mineral samples.